Abstract
Background: Children with Acute Lymphoblastic Leukemia (ALL) in Low Middle income countries(LMIC) face major challenges including treatment abandonment and poor overall survival (OS) and event-free survival (EFS).The preliminary data and results of outcome of Pediatric ALL from our pediatric hematology-oncology center which follows BFM based ALLtreatment protocol has been published in 2015 in ASH forum.Due to non availability of Polymerase chain reaction (PCR)based Minimal residual disease(MRD)analysis, we use multiparametric flowcytometry (FCM) based MRD analysis for remission assessment and risk stratification in our patients. Within resource constraints, we present evidence that outcomes comparable with that seen in high income (HIC)and upper middle income countries(UMIC) can be achieved with a post remission therapy guided by a risk stratification incorporating FCM MRD.
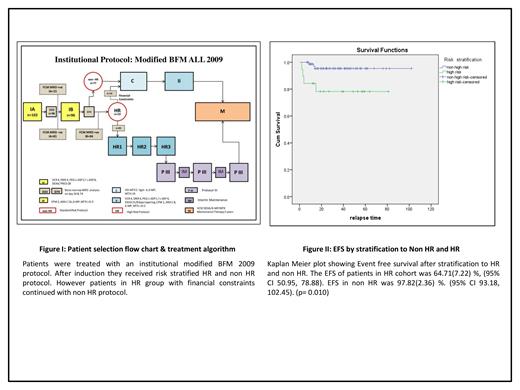
Methods: Following IRB approval,an ambidirectional cohort study was performed using clinical information and outcomes of all patients aged 1to 14 years treated for newly diagnosed B- or T-ALL between January 1,2015 and December 31, 2020. AIEOP BFM ALL 2009 protocol with modifications was followed as the institutional protocol in all patients.The treatment algorithm is mentioned in Figure 1. Patients who underwent multiparametric FCM MRD analysis at the end of Induction IA and whose 6 months follow up details were available were included in the analysis. Patients with Ph positive ALL and those who died during Induction IA were excluded. FCM MRD analysis was performed after Induction IA on Day 33.MRD level above 0.01% was considered positive.MRD assessment was repeated following Induction IB on Day 74 in patients who had MRD positivity on Day 33. At the end of Induction IB patients were risk stratified into High risk(HR) and non High risk(Non HR).HR features included Hypodiploidy(<45chromosomes), Positivity for MLL/AF4 or t(4:11),poor prednisolone response(absolute blast count in peripheral blood ≥1 x 10^9/l on Day 8 of initiation of prednisolone) and non remission on Day33. Patients who had persistent MRD positive on Day74 were reallocated to high risk. Treatment with HR protocol was initiated for high risk patients whenever financially manageable.Statistical analysis was done using SPSS version 21 OS and EFS were assessed by Kaplan-Meier method Patients were censored at last follow-up.
Results: Median follow-up time was 33.5(4-102) months . Study had 102(n=102) consecutive Ph negative patients who underwent induction therapy. Six patients (5.88%) died during induction IA;96(n=96) children who continued treatment were included in further analysis. The mean age at diagnosis was 6(1-14) years .Forty seven (48.95%)patients were male..B-ALL n=84(87.5%) and T ALL n=12.(12.5%). Four patients(4.16%) had CNS disease at diagnosis. Three children (3.13%) had high risk cytogenetics.Ten children (10.42%)in the cohort had poor prednisolone response. Five patients(5.20%) didnot achieve morphological remission on Day33. Fifteen (15.63%) patients were risk stratified as HR during IA. Four more (4.16%) were reallocated to HR in view of persistent MRD positivity after Induction IB. At median follow-up, the OS was 95.35%±2.65(95% CI 90.16-100.54) and the EFS 94.48%±2.74 (95% CI, 89.11%-99.84%).Female gender predicted better EFS (p=0.042).The EFS of patients without CNS disease at presentation was significantly better(93.5% Vs 23.2% p=0.000). The EFS at median follow up of patients in re HR cohort was 64.19% Vs 97.81% in non HR (p=0.010). EFS by risk stratification is shown in Figure II.
Conclusion:Our study suggests that FCM MRD can be successfully incorporated into the treatment algorithm in a resource limited setting. With FCM MRD were able to identify an additional subset of HR patients who otherwise would have been stratified into non HR group. In a prospective cohort , FCM MRD could be tested at an earlier time point in IA induction to facilitate identification of early drug responsive patients with lowest risk of relapse.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal